TECHNOLOGY
We introduce DN Automotive’s technology shown in the best batteries.
-
TECHNOLOGY
-
General Knowledge of Batteries

-
The understanding of Lead-acid Batteries

The understanding of Lead-acid Batteries
Provide knowledge to customers who want to know more about batteries.
-
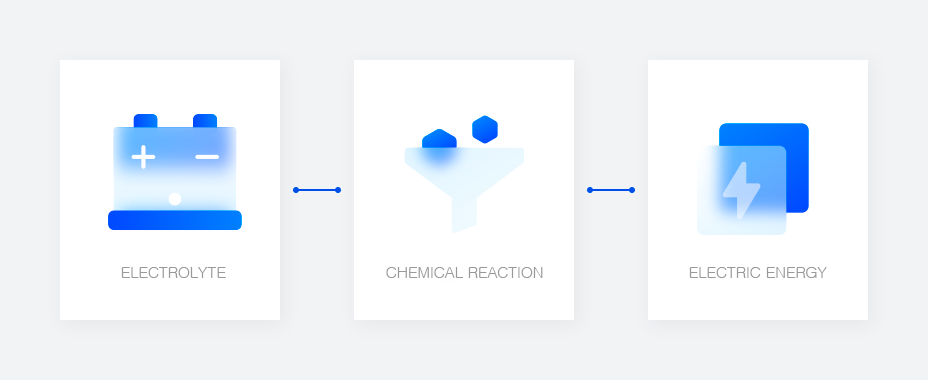
The Definition of a Lead-Acid Battery
A Storage Battery Composed of Lead and AcidThe negative pole which tends to ionize and the positive pole which does not ionize are put together in the electrolyte (thin sulfuric acid concentrated at 37%) to create a circuit and generate electric energy through a chemical reaction.
-
How a Lead-Acid Battery Works
-
Electromotive Force
Electromotive force is the power that generates electricity and pushes it through the electric circuit. The battery generates it by charging and discharging (chemical energy ↔ electric energy). Units are marked V for voltage.
-
Voltage
Voltage is the difference between two electrical energy points in the conductor. It is called an electric potential difference (Voltage/V).
-
Nominal Voltage
Standard voltage specified on the battery → 2V per Cell * The official voltage of car batteries is 12V(2V X 6 Cell)
-
Open Circuit Voltage
This is the voltage when the battery is not electrically connected to the external circuit. It is a stable, open-circuit state created after an hour or more of charging or discharging.
-
Cut-Off Voltage Of Discharge
This is the voltage where the battery discharge must be halted. In usual cases, the voltage plummets after a certain degree of battery discharge. If discharged below a certain point, it over-discharges and damage the battery. Therefore, this is a voltage designated to prevent overdischarge. Please note that the standards for cut-off voltage of discharge differ according to product characteristics and usage. Car battery cut-off voltage of discharge is 10.5V(1.75V Per Cell X 6 Cell) but differs according to pole plate type.
-
Capacity
Capacity refers to the performance of the battery. This is the depleted electricity (discharged electricity X discharged time) when discharging a fully charged battery at a set current to battery cut-off voltage. Units are Ampere Hours (AH). The capacity shows the power of a battery. It is the standard that shows the working capacity of a battery after it is charged.
- - 20 Hour Rate Capacity (20AH)
- It is commonly used for showing battery capacity. It is when a fully charged battery is kept at 25 and continued discharging to battery cut-off voltage(10.5V) at a 20-hour rate current. E.g.) When XP80’s 20 hour rate capacity is 20-hour rate current X time = shown as 80AH →20 hour rate current : 80AH / 20H = 4A → Time for cut-off voltage: 20 hours
- - 5 Hour Rate Capacity (5AH)
- It is commonly used to regulate ignition capacity. It is when a fully charged battery is kept at 25 and continued discharging to battery cut-off voltage(10.5V) at a 5-hour rate current. E.g.) XP80’s 5 hour rate capacity : 5-hour rate current X time = shown as 64AH → 5 hour rate current : 64AH / 5H = 12.8A→ Time for cut-off voltage: 5 hours
-
(Reserve Capacity : Reserve Capacity: R.C) R.C
When the generator breaks down during driving, the minimum electricity required for the vehicle to stay in motion (considering night and harsh weather conditions) is assumed to be 25A. The reserve capacity shows the time in minutes when the battery is discharged down to 25A and continues to battery cut-off voltage(10.5V). E.g.) XP80 has a reserve capacity of 135 minutes
-
(Cold Cranking Ampere : Cold Cranking Ampere: C.C.A) C.C.A
When the battery is kept for 16 hours at -18 (by KS specification regulations), discharged at C.C.A., and the voltage after 30 seconds can be kept at over 7.2V. E.g.) In the case of XP80, it is 630A
-
CCA
- - Actual CCA
- The number measured when discharged according to actual regulated current Higher in larger pole plates. (Large>JIS, EN L>EN LB>N )
- - Mid-CCA
- A theoretical number calculated on a program according to voltage and resistance. Same regardless of size (Large=JIS, EN L=EN LB>N )
-

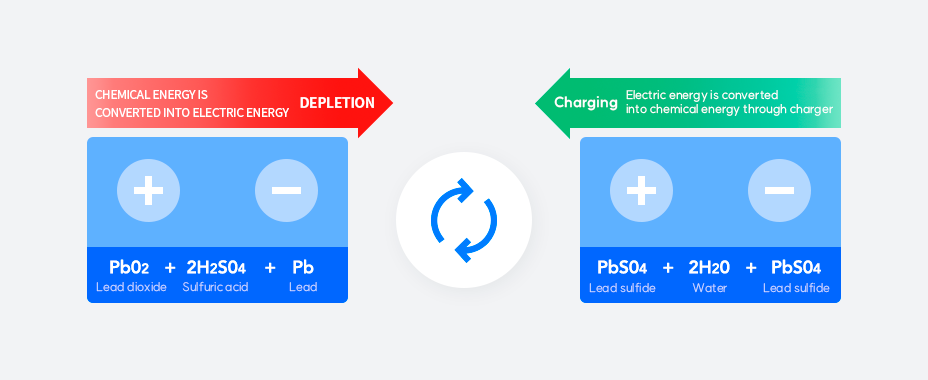
Charging
Charging is a process where the electrolyte’s water turns back into thin sulfuric acid. The specific gravity of the electrolyte and voltage rise to the regulated level. When the battery is fully charged, the specific gravity of the electrolyte and voltage no longer rise. The electric separation of water accelerates, actively generating hydrogen and oxygen. There is active gas generation at the end of charging and when discharging. Hydrogen is explosive and oxygen is flammable, so the battery must be kept away from flame when charging.
-

Discharge
When the battery is discharged, the electrolyte is in the form of thin sulfuric acid (H2SO4 + H2O) almost like water. Only sulfuric acid reacts during the chemical reaction and the specific gravity and voltage of the electrolyte decrease. If the discharge continues, the active material turns into lead sulfate (PbSO4), leading to a state of full discharge that can no longer generate electricity. If the battery is over-discharged below a certain voltage, it can cause shock to the battery and a subsequent life-shortening. This is why, in most cases, car batteries are only discharged to 10.5V (1.75V / Cell).
